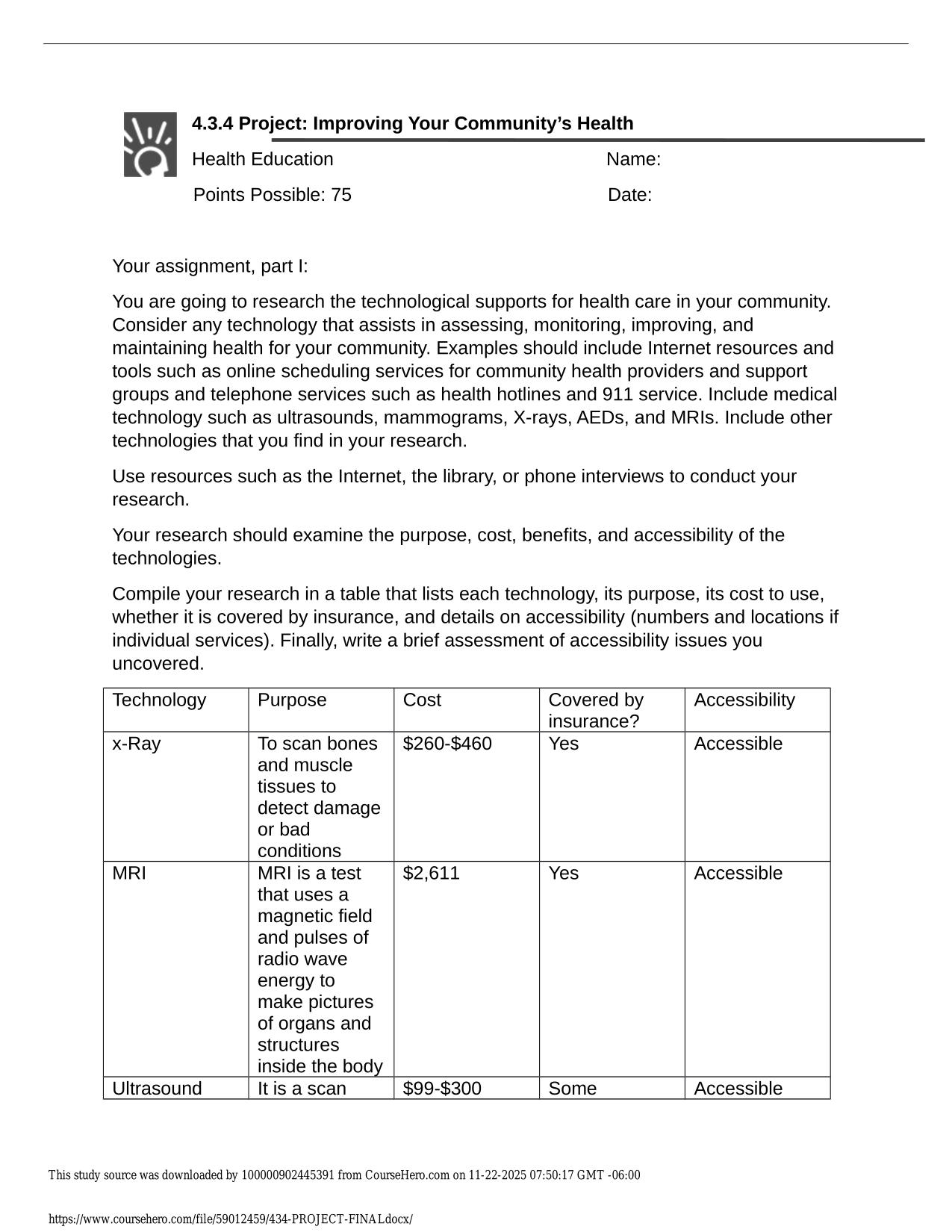
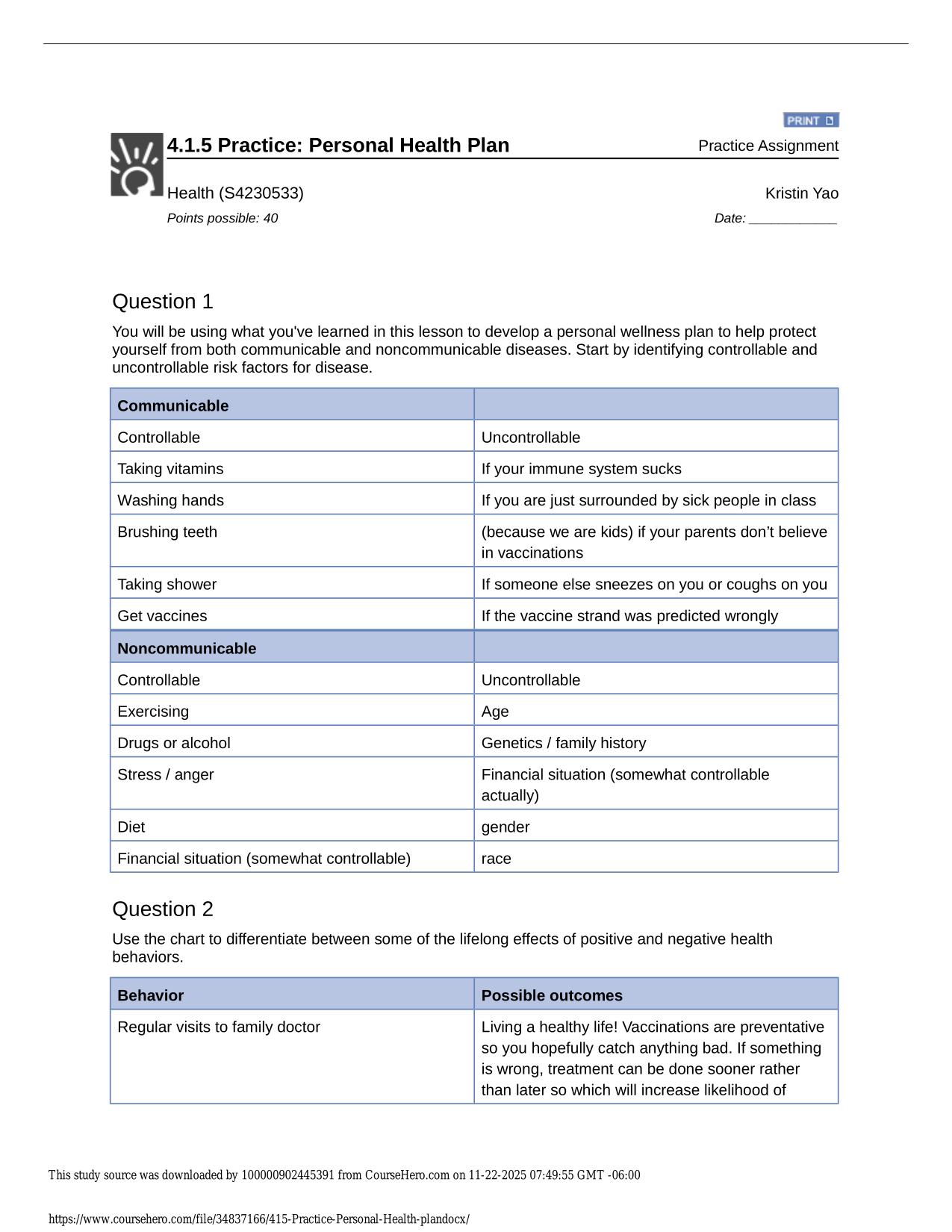
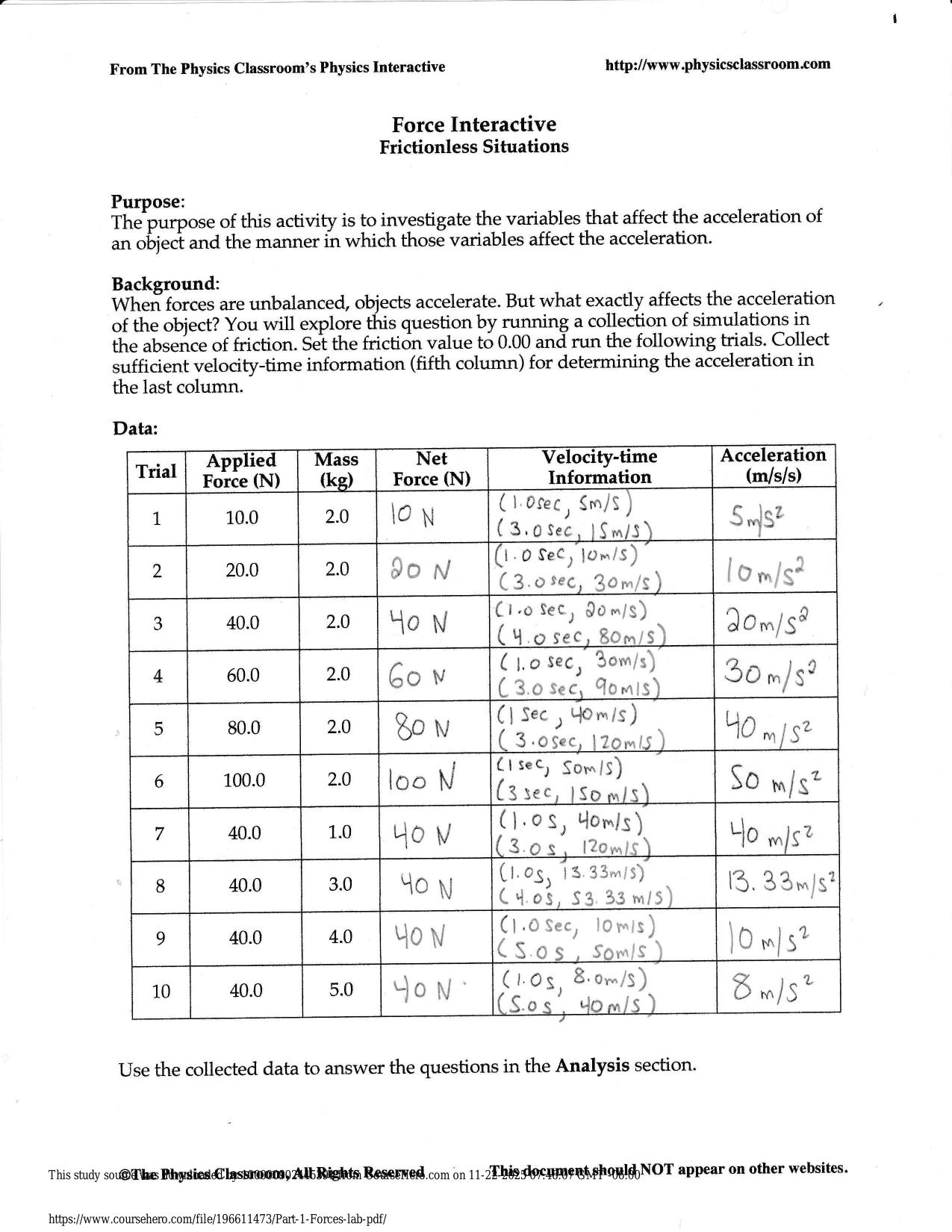
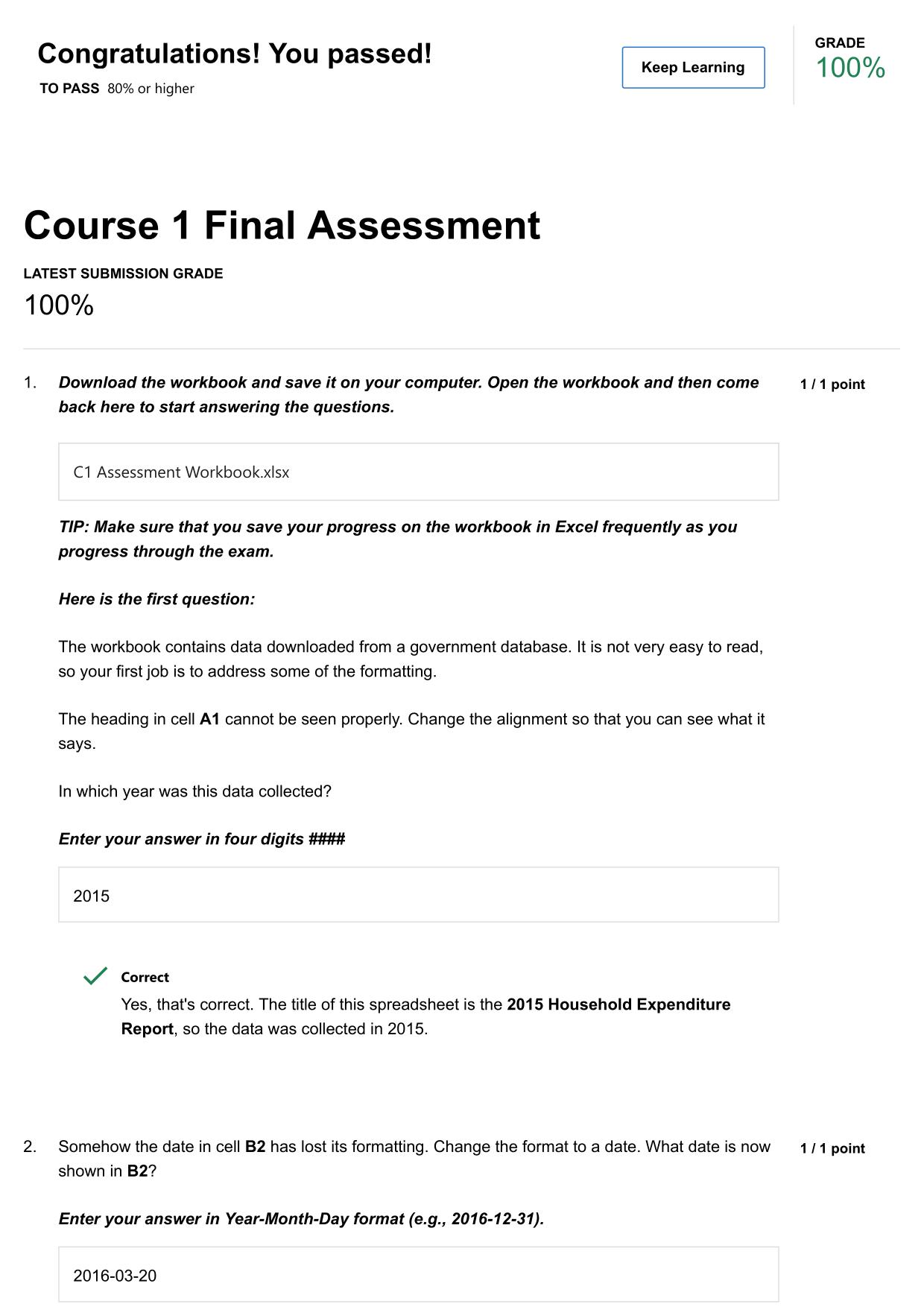
PHM321 FINAL EXAM ACTUAL 2025/2026 UPDATE
Course:
PHM 321
Institution:
PHM 321
PHM321 FINAL EXAM ACTUAL 2025/2026 QUESTIONS AND 100% CORRECT ANSWERS Question: A drug development process involves multiple stages, starting from discovery to post-marketing surveillance. At which phase of clinical research are healthy volunteers pr...
After purchase, you get:
✅ Instant PDF Download
✅ Verified answer explanations
✅ Refund if not Satisfied
✅ Prepared for 2025/2026 test cycle
Overview
Explanations help bridge the gap between theoretical learning and practical application—critical for exam success. You're learning how to take classroom knowledge and apply it to real testing situations. This translation skill is exactly what many students struggle with when facing thorough exams. The practical focus ensures you can actually use what you've learned when it matters most. Inside this resource, candidates will find practice items modeled closely after modern Exam (elaborations) testing standards. The creators have clearly done their homework on current exam trends and question styles. You'll appreciate how each practice session builds your skills in exactly the areas that matter most for your test. It's like having a personal tutor who knows exactly what the examiners are looking for.
Who Is This For?
PHM321 FINAL ACTUAL / UPDATE aspirants aiming to build both conceptual knowledge and exam strategy will find this document extremely useful. Many successful candidates have used similar materials in their preparation. The thorough coverage ensures no important topics are missed. Ideal for motivated learners who want both conceptual clarity and hands-on question practice centered around PHM321 FINAL ACTUAL / UPDATE. Many find it strikes the right balance between theory and application. The integrated approach helps knowledge stick better.
Related Keywords
Detailed Study Description
Frequently Asked Questions
Document Information
| Uploaded on: | October 31, 2025 |
| Last updated: | November 17, 2025 |
| Number of pages: | 279 |
| Written in: | 2025/2026 |
| Type: | Exam (elaborations) |
| Contains: | Questions & Answers |
| Tags: | PHM321 FINAL EXAM ACTUAL 2025/2026 QUESTIONS AND 100% CORRECT ANSWERS Question: A drug development process involves multiple stages, starting from discovery to post-marketing surveillance. At which phase of clinical research are healthy volunteers primarily used to evaluate the drug's safety and tolerability? A) Phase I B) Phase II C) Phase III D) Phase IV - Answer -Correct Answer: A) Phase I Explanation: Phase I of clinical research involves testing the drug on healthy volunteers (20-80 participants). The focus is on safety, tolerability, and determining the maximum tolerated dose. It includes |
Seller Information

AdelineJean
User Reviews (0)
Exam (Elaborations)
$11.00
Add to Cart
100% satisfaction guarantee
Refund Upon dissatisfaction
Immediately available after purchase
Available in Both online and PDF
$11.00
| 0 sold
Discover More resources
Inside The Document
PHM321 FINAL EXAM ACTUAL 2025/2026 QUESTIONS AND 100% CORRECT ANSWERS Question: A drug development process involves multiple stages, starting from discovery to post-marketing surveillance. At which phase of clinical research are healthy volunteers primarily used to evaluate the drug's safety and tolerability? A) Phase I B) Phase II C) Phase III D) Phase IV - Answer -Correct Answer: A) Phase I Explanation: Phase I of clinical research involves testing the drug on healthy volunteers (20-80 participants). The focus is on safety, tolerability, and determining the maximum tolerated dose. It includes single ascending dose (SAD), multiple ascending dose (MAD) studies, and food effect investigations. Question: Which phase of clinical research involves healthy volunteers to assess the safety and maximum tolerated dose of a drug? A) Phase I B) Phase II C) Phase III D) Phase IV - Answer -Correct Answer: A) Phase I Explanation: Phase I studies involve 20-80 healthy volunteers and focus on determining the safety, tolerability, and maximum tolerated dose of the drug. Question: In which phase of clinical research are patients with the disease of interest primarily recruited, with the goal of evaluating the safety and efficacy of a drug? Need assistance on Online classes, Exams & Assignments? Reach out for instant help!! Full Course Assistance, Plagiarism-free Essay Writing, Research Paper, Dissertation, Discussion Posts, etc…. Confidential & Secure services. Tutors are available for all subjects! Email now at: tutorjean01@gmail.com A) Phase I B) Phase II C) Phase III D) Phase IV - Answer -Correct Answer: B) Phase II Explanation: Phase II involves 50-300 patients who have the disease of interest. The focus is on early clinical safety and efficacy evaluation. Question: Which clinical trial phase involves large patient groups (300-3000) and is conducted to confirm the drug's safety and efficacy compared to the standard of care? A) Phase I B) Phase II C) Phase III D) Phase IV - Answer -Correct Answer: C) Phase III Explanation: Phase III trials are conducted with large patient groups (300-3000) to evaluate safety, efficacy, and compare the drug with the standard of care (SOC). Question: What is the primary objective of Phase IV clinical trials? A) To confirm safety and efficacy in healthy volunteers B) To evaluate rare or long-term adverse effects and real-world data C) To compare the drug with standard of care in large patient groups D) To test the drug on patients with the disease of interest - Answer -Correct Answer: B) To evaluate rare or long-term adverse effects and real-world data Explanation: Phase IV involves post-marketing surveillance to detect rare or long-term adverse effects and analyze real-world data. Question: Which phase of drug development involves Post-Marketing Surveillance and may result in the withdrawal or restriction of a drug if adverse effects are identified? A) Phase I B) Phase II C) Phase III Need assistance on Online classes, Exams & Assignments? Reach out for instant help!! Full Course Assistance, Plagiarism-free Essay Writing, Research Paper, Dissertation, Discussion Posts, etc…. Confidential & Secure services. Tutors are available for all subjects! Email now at: tutorjean01@gmail.com
CourseHero & Studypool Unlocks
Get Unlocked CourseHero and Studypool documents files instantly to your email, simply by pasting your link and clicking "Unlock Now". Learn more on how to unlock here.