UCONN ECE BIOLOGY FINAL EXAM ACTUAL 2025/2026 WITH CORRECT ANSWERS
Course:
UCONN ECE BIOLOGY
Institution:
UCONN ECE BIOLOGY
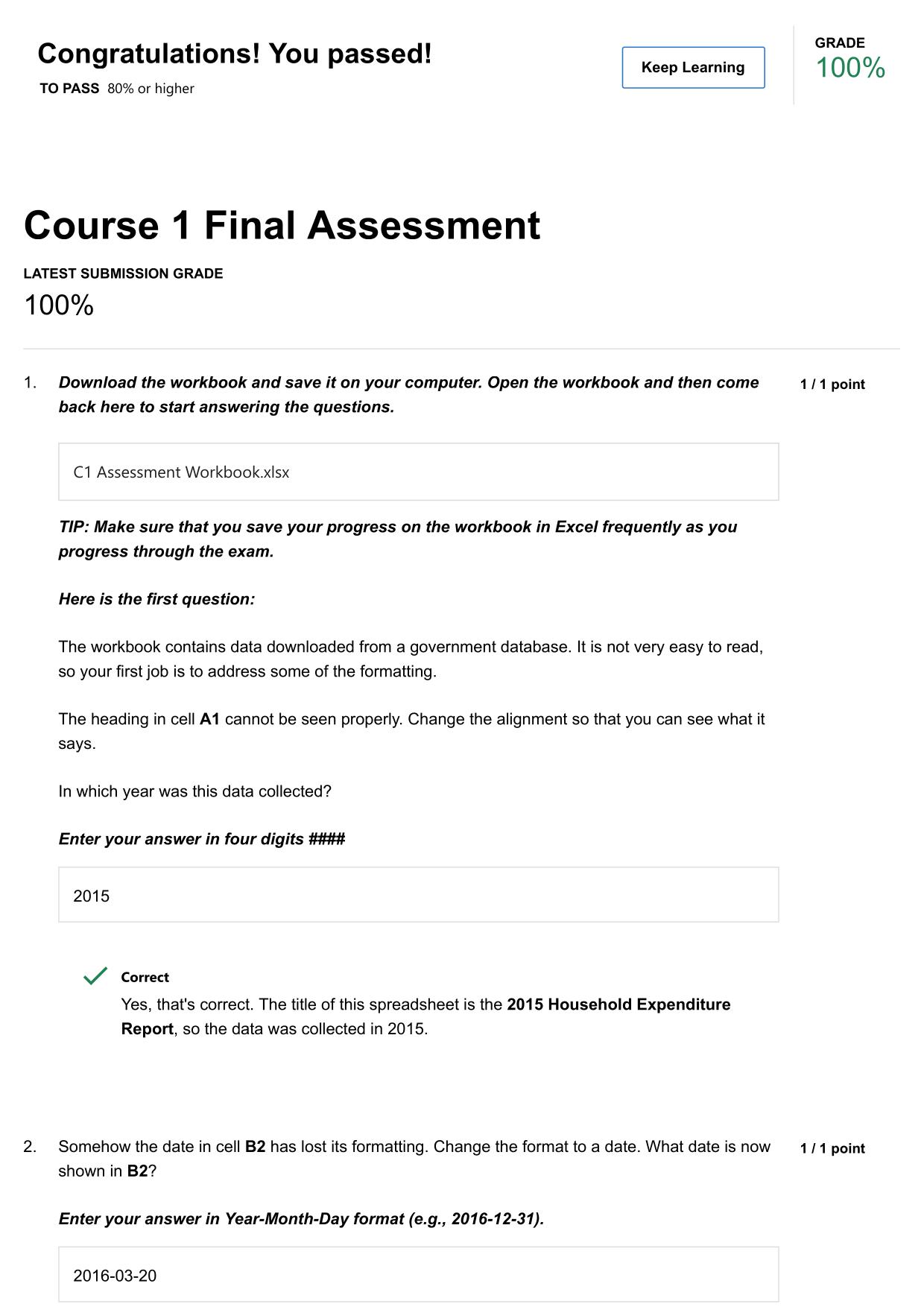
UCONN ECE BIOLOGY FINAL EXAM ACTUAL 2025/2026 QUESTIONS AND 100% CORRECT ANSWERS Ionic - Answer -Ionic bonding is the complete transfer of valence electron(s) between atoms. In ionic bonds, the metal loses electrons to become a positively charged cat...
After purchase, you get:
✅ Instant PDF Download
✅ Verified answer explanations
✅ Refund if not Satisfied
✅ Prepared for 2025/2026 test cycle
Overview
Complex topics are clarified using example-driven reasoning, making challenging areas easier to absorb. Abstract concepts become concrete when you see them applied in practical examples. Students often find that difficult ideas suddenly click when presented through actual scenarios. The example-based approach helps bridge the gap between theoretical knowledge and practical application.
Who Is This For?
Useful for last-minute revision or long-term study plans, especially for those tackling UCONN ECE BIOLOGY FINAL ACTUAL / WITH CORRECT or similar certification paths. Many users keep it bookmarked for quick reference during study sessions. The practical format works well for different study approaches.
Related Keywords
Detailed Study Description
Frequently Asked Questions
Document Information
| Uploaded on: | October 24, 2025 |
| Last updated: | November 17, 2025 |
| Number of pages: | 24 |
| Written in: | 2025/2026 |
| Type: | Exam (elaborations) |
| Contains: | Questions & Answers |
| Tags: | UCONN ECE BIOLOGY FINAL EXAM ACTUAL 2025/2026 QUESTIONS AND 100% CORRECT ANSWERS Ionic - Answer -Ionic bonding is the complete transfer of valence electron(s) between atoms. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. Ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal Ex. Sodium Chloride (NaCl) |
Seller Information

AdelineJean
User Reviews (0)
Exam (Elaborations)
$9.50
Add to Cart
100% satisfaction guarantee
Refund Upon dissatisfaction
Immediately available after purchase
Available in Both online and PDF
$9.50
| 0 sold
Discover More resources
Inside The Document
UCONN ECE BIOLOGY FINAL EXAM ACTUAL 2025/2026 QUESTIONS AND 100% CORRECT ANSWERS Ionic - Answer -Ionic bonding is the complete transfer of valence electron(s) between atoms. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. Ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal Ex. Sodium Chloride (NaCl) Covalent - Answer -Covalent bonding is the sharing of electrons between atoms. This type of bonding occurs between two atoms of the same element or of elements close to each other in the periodic table. Ex. Water (H2O) Hydrogen - Answer -Weak bond between a slightly positive atom of a polar covalent bond attracted to a slightly negative atom in a polar covalent bond Ex. bond between water molecules Van der Waals - Answer -Weak attractions that allow transient partial charges Need assistance on Online classes, Exams & Assignments? Reach out for instant help!! Full Course Assistance, Plagiarism-free Essay Writing, Research Paper, Dissertation, Discussion Posts, etc…. Confidential & Secure services. Tutors are available for all subjects! Email now at: tutorjean01@gmail.com Ex. diatomics (HH) What is allosteric regulation and how does it work? - Answer -Regulating the availability of the enzymes active site Can change structurally (inhibit) or hold open for better binding (enable) What factors affect how permeable a phospholipid membrane is to a molecule? - Answer -- Change in temp - pH - Number of saturated and unsaturated fatty acid tails - Number of proteins What are the reactants/products of glycolysis? - Answer -R: 1 glucose molecule P: 2 pyruvate 2 Net ATP 2 NADH What is the difference between the energy investment phase and the energy payoff phase? - Answer -Investment: 2 ATP are needed to initiate the first half of reaction Payoff: Creating 4 ATP by substrate level phosphorylation Why is the energy investment phase needed? - Answer -To get the reactions started Amino Acid - Answer -R group OH group H Need assistance on Online classes, Exams & Assignments? Reach out for instant help!! Full Course Assistance, Plagiarism-free Essay Writing, Research Paper, Dissertation, Discussion Posts, etc…. Confidential & Secure services. Tutors are available for all subjects! Email now at: tutorjean01@gmail.com
CourseHero & Studypool Unlocks
Get Unlocked CourseHero and Studypool documents files instantly to your email, simply by pasting your link and clicking "Unlock Now". Learn more on how to unlock here.